Another UNM Doctor Confirms Intimidation for Raising Concerns About Use of IRRAflow Device
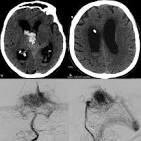
Despite UNM’s Silence, Another Communication Surfaces Relative to Intimidation of Medical Personnel For Questioning Use of a Medical Device in Neurosurgery.
Late last year, The Candle began publishing a series of stories about bullying behavior, discrimination and harassment towards medical students, residents, and faculty – behavior that was included among the reasons UNM’s School of Medicine had lost accreditation for its Neurosurgery Residency program in 2019.
Despite eventually regaining its accreditation in 2022, it appears there are still many issues the School of Medicine is facing relative to how the Neurosurgery department is run – especially since 2023.
The series has also focused on concerns a group of medical professionals raised about patients, or their advocates, not being properly informed by UNM personnel when it came to seeking their consent in the use of a medical device known as the IRRAflow.
In addition to the matters surrounding the consent issues they raised, doctors and support personnel expressed frustration with leaders of the School of Medicine and the Neurosurgery department for not taking seriously the potential, and unnecessary, damage done to a significant number of neurosurgery patients due to the inappropriate use of the IRRAflow device.
The Candle reported that in January of 2024, Neurosurgery department leaders were made aware that at least one of three clearly identified and significant adverse events (SAE) related to the misuse of the medical device likely resulted in an abnormal inflow of fluid causing a dangerous buildup of pressure on the brain tissue.
The patient never recovered after experiencing neurologic decline.
In addition to the event referred to above, other examples of the problematic use of the medical device have also been brought to the attention of the UNM leadership in 2023 and 2024.
Christian Ricks, MD, (Assistant Professor, and Residency Program Director of SOM – Neurosurgery), Chad Cole, MD, (Associate Professor, Vice Chair of Clinical Operations for Neurosurgery), Peter Shin, MD, (Dr. Shin served as Interim Chair of Neurosurgery and Residency Program Director), and John Russell, MD, (professor and former longtime chair of The University of New Mexico Department of Surgery, and until January of 2025, acting Chair of Neurosurgery), have been made aware of significant adverse events and other instances of the misuse of the device.
More recently The Candle was informed of a communication between a doctor and a leader of the Neurosurgery department expressing concerns about being asked by another department leader to place an IRRAflow in a patient experiencing bleeding in a confined space of the brain with severe symptoms, significantly increasing the risk of further brain damage, and potential herniation.
According to medical descriptions, such conditions of a combination of AVM bleeding in a confined space (Arteriovenous malformations, AVMs, are abnormal tangles of blood vessels that can bleed, causing neurological damage), with severe symptoms, significantly increases the risk of further brain damage, hydrocephalus, herniation, and ultimately, can lead to death.
According to medical personnel at UNM, there have been more than 110 instances of IRRAflows being placed in patients.
The same sources have told The Candle, when they have raised concerns about the manner of use of the IRRAflow devices, leaders of the Neurosurgery department have threatened retaliation, including termination as an employee of UNM.
It is significant that the most recent information relative to an internal communication of concern regarding the use of the IRRAflow is about an event that occurred about four months after Neurosurgery leaders were previously warned about the problems with the device – including an adverse event involving a patient who died.
For several months, The Candle has requested access, via an Inspection of Public Records Act request, to view records of adverse events, including deaths, experienced by patients of the hospital. Each of our requests included the acknowledgement that UNM make appropriate redactions of information that would prevent the revealing of a patient’s identity.
UNM refuses to provide any information, even as to the number of events.
The Candle plans to expand on this reporting in a few days, with a report on inappropriate treatment of medical personnel and pay disparities within the hospital.
UNM’s Relationship With IRRAS
In our previous coverage, The Candle reported on the relationship between UNM (along with one of its former Neurosurgery leaders and researchers, Dr. Andrew Carlson), and the IRRAS corporation – the company that manufactures, distributes and sells the IRRAflow device and its components.
This following is from the story The Candle published on January 24, 2025:
A University of New Mexico Hospital Team (led by UNM School of Medicine’s former Assistant Professor Andrew Carlson, MD) was identified as a “Valued Partner” by IRRAS, the developer and marketer of IRRAflow – the device referred to above.
As reported earlier, the IRRAflow device represents a major investment by IRRAS, which expects a return on its investments.
Since at least 2019, through part of 2024, Carlson developed assessments of the success of the device, and had his team publish their assessments in medical journals.
Such publications have had a buttressing effect for IRRAS’ roll out of production and sales of the IRRAflow device.
IRRAS writing about the type of efforts of its “valued partner” (Carlson’s UNM Team) in late 2022, “It is important for our team to increase the rate of product adoption, and the generation of impactful clinical data will play a key role in this effort.“ [Emphasis added.]